Wednesday's at 3.00pm (unless otherwise stated)
Colloquia will usually be held in JA3.14
John Anderson Building
107 Rottenrow, Glasgow
Coffee and Tea served at 4.00 pm.
All Welcome
Coordinated with the Colloquia at the Department of Physics and Astronomy of the University of Glasgow. (They may have donuts but we have free chocolate covered biscuits and coffee!)
Colloquia Schedule 2017-2018
Semester I
- 20/09/17 - Oliver Henrich (Dept of Physics, University of Strathclyde)
- 01/11/17 - Sebastian Van De Linde (Dept of Physics, University of Strathclyde)
- 08/11/17 - Nick Kelly (Dept of Mechanical and Aerospace Engineering, University of Strathclyde) *
- 14/11/17 - Peter Mulser (TQE: Theoretical Quantum Electronics, Tech. Univ. Darmstadt) *
- 15/11/17 - Frederique Vanholsbeeck (The University of Auckland, New Zealand)
- 01/12/17 - Robert Fedosejevs (University of Alberta, Edmonton) *
Semester II
- 08/01/18 - Sebastian Munck (VIB-KU Leuven Center for Brain and Disease Research, Belgium) *
- 24/01/18 - Alex R. Jones (Biometrology Group, National Physical Laboratory)
- 31/01/18 - Lucy M. Collinson (The Francis Crick Institute)
- 28/02/18 - Ferdinand Scholz (Ulm)
- 14/03/18 - Fred Manby (University of Bristol)
- 21/03/18 - Nasr Hafz (Shanghai Jiao Tong University) *
- 12/04/18 - Thomas Heine (TU Dresden, Germany) *
- 18/04/18 - Carla Molteni (King’s College London) *
- 19/04/18 - Jonathan Nylk (University of St Andrews) *
- 26/04/18 - Massimo Giudici (Institute de Physique de Nice) *
- 03/05/18 - G. Ravindra Kumar (Tata Institute of Fundamental Research, Mumbai) *
- 31/05/18 - Erdinc Sezgin (Weatherall Institute of Molecular Medicine, University of Oxford) *
* Note: Outside of regular schedule.
Mesoscopic Simulation of Soft Condensed Matter
Oliver Henrich (Dept of Physics, University of Strathclyde) 20 September 2017, JA3.14, 3pm
Soft condensed matter is an interdisciplinary field at the interface of physics, chemistry and biology. It comprises a huge variety of materials that we know from our everyday lives, for instance colloidal suspensions, glasses, amphiphilic mixtures, polymeric liquids, foams, gels, granular matter, liquid crystals, and a vast number of biologically relevant substances.
All these materials share the common feature that their generic physical behaviour occurs at energy scales that are comparable to thermal energies at room temperature, which is why we call them ‘soft’. As deformability often entails flow, hydrodynamics plays a particularly important part in their dynamical behaviour.
Another characteristic feature is their propensity to self-assemble into more complex structures on intermediate time and length scales that are well above the molecular level, but also much smaller than the macroscopic lab scale. Both aspects have led to the development of specialised, mesoscopic simulation methods for soft matter.
Starting from Landau’s phenomenological theory of a binary, phase-separating fluid, I will outline a thermodynamically consistent description of complex fluids that is based on so-called free energy models. I will introduce into the lattice Boltzmann method, which solves a discretised version of the Boltzmann equation from statistical mechanics, and show how these concepts can be applied to study novel composite materials for tuneable optical filters and encryption devices.
Single-molecule based super-resolution imaging
Sebastian Van De Linde (University of Strathclyde) 1 November 2017, JA3.14, 3pm
Fluorescence microscopy is the method of choice to study biological samples in a comparatively non-invasive way. The field has received a powerful boost with the development of super-resolution imaging methods, which overcome the limitations imposed by optical diffraction.
A very strong class of these novel techniques can be merged under the generic term single-molecule localization microscopy (SMLM), which relies on the detection of single-molecules, their precise localization and the reconstruction of an artificial super-resolution image. It can improve on the ~200 nm resolution limit by a factor of ten and more, and thus have opened the door for the study of finer cellular ultrastructure.
While in the beginning the driving force in SMLM was methodological advancements, such as development and characterization of fluorophores, multicolour and 3D imaging capabilities, in recent years SMLM is increasingly applied in biology and medicine. Besides its high spatial resolution SMLM also provides access to quantitative information and thus enables the study of structure-function relationships.
I will provide a concise overview of the development and underlying principles of SMLM with an emphasis on dSTORM, describe novel technical developments and conclude with applications in the field of neurobiology.
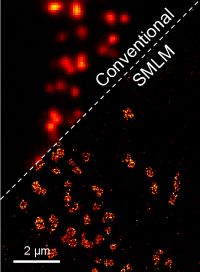
Fig. 1 Super-resolution image of active zones in Drosophila, comparison of conventional wide-field fluorescence image and SMLM.
Introducing the New Energy Strategic Theme and Exploring Collaborative Opportunities
Nick Kelly (Mechanical and Aerospace Engineering, University of Strathclyde) 8 November 2017, JA5.05, 3pm
The talk will provide an overview of the University’s Energy Strategic Theme, detailing the theme objectives; the various areas of energy research activity covered; funding opportunities and upcoming activities. There will also be an opportunity for discussion, to identify possible areas of collaboration between Physics and other university energy researchers and how these could be taken forward.
Adiabatic invariants and generalized ponderomotive force
Peter Mulser (TQE: Theoretical Quantum Electronics, Tech. Univ. Darmstadt) 14 November 2017, JA 3.14, 1pm
The ponderomotive force embraces a realm of physics: collisionless shock wave generation, novel schemes of particle acceleration, stimulated Brillouin and Raman scattering in solids, liquids, gases and plasmas, ac Stark effect in atoms, and the Lamb shift. In the introduction the standard expressions of the ponderomotive force, or wave pressure, are presented and their derivations and physical interpretations are indicated.
In the central part of the talk its connection with the adiabatic invariants is established. Conservation of action is illustrated by various, also historical examples to begin with Lord Rayleigh, and are verified by simple arguments. The adiabatic theorem is formulated and its connection with the Poincaré-Cartan invariant is shown. On this Hamiltonian basis we are prepared for generalized expressions of the ponderomotive potential and force. A fundamental difference between the forces from a longitudinal and a transverse wave will appear. Adiabatic particle trapping, detrapping and acceleration in the electron plasma wave will result as a byproduct. The results will be illustrated by simple case studies.
In the conclusion a survey on conservation of action in various disciplines of physics and applied mathematics: in optics, thermodynamics, quantum physics, time scale methods and linearization, will be briefly discussed. The principle unifying them lies in one property of alternating series of mathematics.
Biophotonics at the University of Auckland
Frederique Vanholsbeeck (The University of Auckland, New Zealand) 15 November 2017, JA3.14, 3pm
Our research projects focus on different imaging modalities such as fluorescence quantification using the optrode, an all-fibre real time spectroscopic optical probe and optical coherence tomography (OCT) imaging.
OCT is a non-invasive imaging technique based on low coherence interferometry that provides high resolution 3D images of samples with a depth range of a few mm. The depth resolution is inversely proportional to the light source bandwidth and can be as good as 1 µm in some recent experiments. Recently, we have been working on new swept sources to get a better imaging depth. We also use these sources to map chromatic dispersion in tissue in order to differentiate tissue type. Lately, we have been working on polarisation sensitive OCT to detect early signs of osteoarthritis.
We are using spectroscopic fluorescence to detect and classify bacteria or monitor their activity. This work has a particular focus on food safety where bacterial enumeration is important.
During the talk, I will present our recent results on these topics obtained by the University of Auckland biophotonics group.
High Intensity Laser-Plasma Interactions in the Relativistic Regime
Robert Fedosejevs (Department of Electrical and Computer Engineering, University of Alberta, Edmonton) 1 December 2017, JA3.14, 2pm
Using Chirped Pulse Amplification (CPA) technology it is now possible to amplify laser pulses to instantaneous power levels of several petawatts in ultrashort pulses with durations of 20 to 1000 fs. Facilities exist and are being built with such capabilities around the world, opening up a range of exciting and challenging opportunities in particle acceleration, tunable x-ray sources and table top nuclear physics sources. The focused intensities currently range from 1018 W/cm2 to 1022 W/cm2 where the motion of electrons in the EM laser field is strongly relativistic. Applications range from 100 MeV class ion sources for cancer therapy to ignition sources for laser fusion energy. Recently, we have been involved in studies of MeV electron generation for applications in Fast Ignition Fusion Energy and the study and development of laser wakefield acceleration sources of electrons up to the GeV energy level. These studies have been carried out at the Titan Laser Facility in Lawrence Livermore National Laboratory and using 200 TW laser facilities located at INRS in Montreal, Canada, and at CLPU in Salamanca, Spain. The wakefield accelerated electrons, in turn, generate synchrotron-like betatron radiation which can be used as a broadband femtosecond x-ray probe pulse. We have applied these ultrashort probe pulses to study the ionization dynamics of warm dense solid aluminium at electron temperatures of 20 eV to 30 eV, using x-ray k-shell absorption spectroscopy. Results from a number of these experiments will be presented and discussed.
Analysing the distribution patterns of membrane constituents on the cell plasma membrane based on inhomogeneity
Sebastian Munck (VIB-KU Leuven Center for Brain and Disease Research, Belgium) 8 January 2018, JA3.26, 11am
The plasma membrane is the outer limit of the cell and plays an essential role in signal transduction, transport of molecules, and adhesion to substrates or other cells. Plasma membrane constituents, proteins, and lipids are organized in complex organizations ranging from random to clustered, as part of signaling platforms during dynamic events including endocytosis or distribute into gradients on polarized cells. These distributions are key to multiple signaling events at the cell surface, including migration, differentiation, proliferation, and extracellular stress.
We have developed an approach that allows quantifying the overall spatial organization of proteins and lipids on the cell surface and that, unlike current tools, is compatible with the diverse organization types of the cell membrane, including polarity. To quantify the distribution of plasma membrane constituents, we make use of a tool called tessellation to divide the space surrounding each detected membrane component. Consequently, we quantify the distribution of areas surrounding the identified constituents to describe various spatial patterns in degrees of inhomogeneity. We have integrated an intensity-based correction to be able to analyze images stemming from devices with a wide range of resolutions. We call our method Quantitative Analysis of the Spatial distributions in Images using Mosaic segmentation and Dual parameter Optimization in Histograms (QuASIMoDOH). We demonstrate that QuASIMoDOH can be used to assess protein and lipid patterns, quantifying distribution changes and spatial reorganization at the cell surface. An ImageJ/Fiji plugin of this analysis tool is available.
Animal magnetoreception – a non-trivial effect of quantum mechanics on biology?
Alex R. Jones (Biometrology Group, National Physical Laboratory), 24th January 2018, JA3.14, 3pm
Many animals can sense the Earth’s magnetic field (MF) to aid with behaviours such has migration, but the biophysical origin of this remarkable ability has confounded scientists for decades. Considerable evidence indicates the primary magnetoreceptor is the flavoprotein, cryptochrome (CRY), which has been proposed to provide geomagnetic information via a quantum effect of MF on photochemical radical pair intermediates.
Although the sub-atomic description of any biological system necessarily encounters the quantum domain, it has been argued that in the vast majority of cases these quantum effects are incidental or ‘trivial’. Why bother with a quantum description of the macroscopic world of biology when invariably a classical one will suffice?
The radical pair model of animal magnetoreception might be different, however. Research to elucidate its biophysical basis encompasses almost the entire spectrum of scientific endeavour, from theoretical quantum mechanics to animal behaviour. In this lecture I will give an overview of this field of research, with particular focus on my own interest in the interplay between CRY structure and the transduction of magnetic signals. In doing so, I hope to go some way to answering the question: is animal magnetoreception an example of a non-trivial effect of quantum mechanics on biology?
Correlative Imaging: From Cells to Stars
Lucy M. Collinson (The Francis Crick Institute) 31 January 2018, JA3.14, 3pm
Correlative light and electron microscopy (CLEM) combines the benefits of fluorescence and electron imaging, revealing protein localisation against the backdrop of cellular architecture. The correlative imaging field is expanding rapidly, and encompasses workflows that link many different imaging modalities, to answer scientific questions in the biological and physical sciences. We link fluorescence microscopes (widefield, confocal, super-resolution and light-sheet) with electron microscopes (scanning, transmission, serial block face and focused ion beam) and X-ray microscopes (microCT and soft X-ray) to analyse a range of biological samples, from single cells to whole model organisms.
Our technology development work has focused on improving the speed, accuracy and accessibility of CLEM. During this development work, it became clear that the technical challenges associated with correlative imaging are exaggerated when working in 3D. To increase protein localisation precision, we developed an ‘In-Resin Fluorescence’ (IRF) protocol that preserves the activity of GFP and related fluorophores in resin-embedded cells and tissues. The sample preparation is relatively fast, and also introduces electron contrast so that cell structure can be visualised in the electron microscope. Once the resin blocks have been cut into ultrathin sections, out-of-plane fluorescence is removed resulting in physical ‘super-resolution’ light microscopy in the axial direction, which increases the accuracy of the LM-EM overlays. Localisation precision is further increased by imaging the IRF sections in vacuo in the next generation of commercial integrated light and electron microscopes (ILEM). We were able to further improve accuracy by developing integrated super-resolution light and electron microscopy, using the remarkable blinking properties of GFP and YFP in-resin in vacuo.
With the advent of dual contrast samples comes the potential to locate and track fluorescent cells during sample preparation and automated 3D EM image acquisition. We designed and built two new locator tools – a fluorescence microscope designed to integrate with an ultramicrotome to locate cells during trimming and sectioning (the ultraLM), and an even smaller version that fits into the extremely tight space of the SBF SEM vacuum chamber for on-the-fly tracking of fluorescent cells during long automated imaging runs (the miniLM).
As electron microscopes become more automated, data outputs are increasing astronomically, and so the bottleneck in our work is shifting from data acquisition to data analysis. I will describe the ideas and workflows we are developing to deal with big data, and describe our Citizen Science collaboration with the Zooniverse platform, which is helping us to develop automated detection and segmentation of cell structures in EM images.
Group-III-nitride-based hetero structures for sensor and UV-LED applications: Research activities at Ulm University
Ferdinand Scholz (Ulm) 28 February 2018, JA3.14, 3pm
GaN and related compounds have attracted immense research interest world-wide since the first presentation of very bright blue LEDs in 1992. At Ulm University, we have focused on the epitaxial growth and characterization of GaN-based hetero structures for optoelectronic devices since more than 20 years, contributing in particular to studies of semipolar LED structures over the last 10 years. Currently, we focus our efforts on two other topics which will be taken as main subjects for this talk.
Owing to the excellent chemical stability of GaN, such materials are regarded as bio-compatible and hence they are good candidates to act as chemical and bio-medical sensors. In our current studies, polar GaInN quantum well structures are applied for sensing different molecules adsorbed on the sample surface. Adsorbate-caused changes of the band bending near the surface and hence the photoluminescence signal of near-surface GaInN quantum wells are taken as chemical sensing signal. This optical read-out makes electrical contacts obsolete, which might be problematic in chemically harsh environments. The sensitivity depends on the design of the hetero structures like QW and cap layer thickness, as evaluated by band structure simulations. Besides gases such as oxygen and hydrogen, also biomolecules can be adsorbed on the semiconductor surface and studied by PL. As an example, we have studied the protein “ferritin”. Its concentration in blood is an important indicator for the iron storage state in our body. Ferritins with and without iron-load (the latter corresponds to apoferritin) are immobilized on hydroxylated polar GaInN quantum well surfaces and lead to a distinct spectral shift of the quantum well PL. In order to selectively detect such proteins, we investigate possibilities to functionalize the GaN surface by other specific organic molecules.
Another focus of our work is set on AlGaN-based hetero structures for LED applications in the UV-C spectral range (around 270 nm). Such LEDs still suffer from relatively poor external quantum efficiencies, which is discussed to be caused at least partly by the fairly large lattice mismatch between the various materials forming these hetero structures. Therefore, we investigate the incorporation of boron (B) into AlGaN layers, which reduces the lattice constant and hence may help to manage the strain in such hetero structures. Unfortunately, the miscibility of B in AlGaN is very low. For total boron contents of about 5% in AlBN epitaxially grown by MOVPE, we see strong indications for phase separation between AlN and boron. For lower boron contents, phase separation could not be observed in thin layers. However, strong 3D growth developed for thicker or multi layers (Fig. 1) resulting in tilted facets and surface roughening, certainly a consequence of the low mobility of B on the growing surface. Hence, further optimization of the MOVPE growth conditions has to be done.
These studies are done in cooperation with K. Thonke et al., Inst. of Quantum Matter, T. Weil et al., Inst. of Organic Chemistry III, and U. Kaiser et al., Central Facility of Electron Microscopy, all at Ulm University.
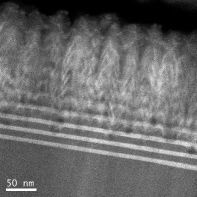
Fig.1: Cross-section Transmission electron micrograph of an AlN/AlBGaN superlattice structure.
Quantum mechanics of light-matter and system-bath interactions in photosynthesis
Fred Manby (University of Bristol) 14 March 2018, JA3.14, 3pm
Almost all of the biomass in the world derives from the harvesting of solar energy through photosynthesis. A great deal is known about the structure and dynamics of the machinery responsible for this process, but mysteries remain. Photosynthesis in purple bacteria is amazingly efficient: chemical change is induced almost every time a photon is absorbed. This is curious because it implies very efficient transport of the energy through a disordered system. Here we will explore the quantum mechanics of the light-matter interaction, and of the energy-transport processes involved, to try to arrive at a clear picture of how photosynthetic light harvesting really works.
Recent Progress on laser-plasma acceleration and radiation generation experiments
Nasr Hafz (Shanghai Jiao Tong University) 21 March 2018, JA5.06, 3pm
Laser-plasma particle acceleration is a non-traditional acceleration technique that could lead to a significant downsizing of future high-energy accelerators [1-2]. Recently, we have conducted laser-plasma electron acceleration experiments in Shanghai, China. Experiments were based upon a newly-installed 200 TW, 30 fs Ti:sapphire laser. We have performed several laser wakefield acceleration (LWFA) experiments:
- based on the self-injection of electrons from a 4 mm long He plasma interacted with 30 TW laser pulses (a0 ~1.2). We observed ~120 MeV electron beams with ~ 40% energy-spread [3].
- To improve the electron beams we have conducted a second set of experiments, where we employed the ionization-injection mechanism and observed a significant enhancement of the beam energy up to 400 MeV with a reduction of the energy-spread to 4% [4].
- A follow up experiment employed ~ 120 TW laser pulses and 1 cm-scale plasma to investigate ionization injection to boost the electron energy and quality. Here we observed narrow energy-spread beams (7%) with 1.2 GeV peak energy; see Figure below [5]. 3D-PIC simulations verify the experimental results and the physics.
- Additionally, we have measured the emission of synchrotron x-rays from the accelerated relativistic electrons where hard x-ray beams with few mrad divergence and energies up to 100 keV have been observed [6].
- Finally, we have observed electron-position pairs with energies of 75 MeV and 10 MeV using gamma ray beams using bremsstrahlung radiation [7].
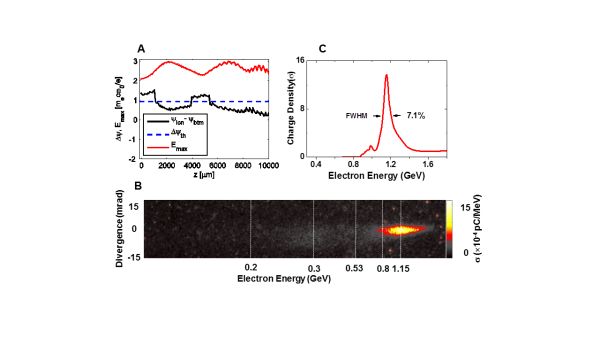
Figure. High-quality GeV electron beam generated by laser-plasma at Shanghai Jiao Tong University.
References
- T. Tajima and J. Dawson, Phys. Rev. Lett. 43, 267 (1979).
- N. Hafz et al., Nature Photonics 2, 571 (2008).
- S. Li, N. Hafz* et al., J. Appl. Phys. 116, 043109 (2014).
- S. Li, N. Hafz* et al., Opt. Express 22, 29578 (2014).
- M. Mirzaie, S, Li, M. Zeng, N. Hafz* et al., Scientific Reports 5, 14659 (2015)
- K. Huang, et al. N. Hafz, et al., Scientific Reports 6, 27633(2016)
- N. Hafz et al., papers under preparation (2018)
Hydrogen isotope separation in metal-organic frameworks and layered materials
Thomas Heine (Chair of Theoretical Chemistry, TU Dresden, Germany) 12 April 2018, JA3.14, 3pm
Heavier hydrogen isotopes are rare in nature, but they have a strong effect for our daily life: deuterium (D) serves science to understand chemical reactions and is used in modern drugs to enhance their activity, while tritium (T) is both a radioactive contaminant (one of the main ones in the Fukushima disaster), but also a prospective fuel for future fusion plants and source of precious 3He. To date, no efficient technology that can capture heavy hydrogen isotopes is available, nor is it possible to detect T contamination in low concentrations. In this talk, I will highlight recent progress in hydrogen isotope separation possibilities which exploit nuclear quantum effects as means for separation. Those exploit either the different size of the isotopes (quantum confinement), or the difference in zero-point energy if hydrogen is adsorbed by host structures.
In this talk I will show how these effects can be exploited in metal-organic frameworks, materials where molecules are stitched together by strong bonds to form regular crystals, which adsorb dihydrogen isotopologues, and by diffusion in the interstitial area in layered materials h-BN and MoS2. Interestingly, in all these examples quantum effects remain important at elevated temperature of 77K or higher, or even at room temperature.
Order-disorder Interplay in Nanocrystals under Pressure
Carla Molteni (King’s College London) 18 April 2018, JA3.14, 3pm
Nanocrystals show a wealth of distinctive behaviours with respect to their bulk counterparts, which can be tuned by varying their size, shape and surface. These include the way they respond to applied pressure, transforming from the original crystalline structure to new ordered or disordered phases. Of particular technological and fundamental interest are nanocrystals of tetrahedrally coordinated materials, such as Si, Ge, CdSe, CdS and ice, which can be driven by pressure toward highly coordinated crystalline or amorphous phases. We have used a series of simulation techniques, including density functional theory, molecular dynamics and the enhanced sampling method metadynamics, to characterize the mechanisms of structural transformations in these nanocrystals, focussing on the competition between amorphization and crystallization, the effects of an implicit or explicit description of the environment and the emergence of metallic phases. Moreover, we have investigated, as a function of size and surface ligands, pressure-induced effects in the electronic and optical properties of nanocrystals, which may be exploited in the development of novel pressure sensors.
Shaped Photonics for Biomedical Imaging
Jonathan Nylk (School of Physics & Astronomy, University of St Andrews) 19 April 2018, JA3.26, 12pm
Beam shaping is a suite of techniques that is continually expanding the optical imaging domain. Advances in structured illumination techniques are extending beyond the traditional diffraction limit and similar methods in optical coherence tomography are pushing the limits of deep tissue imaging, traditionally the remits of electron microscopy and ultrasound/MRI imaging respectively. I will discuss our recent work in the application of shaped photonics approaches to light-sheet microscopy (LSM) for imaging across scales.
LSM is revolutionising biomedical imaging, particularly for neuroscience and developmental biology. High-speed, high-contrast, and minimally phototoxic imaging is achieved by illuminating only a thin plane of the specimen and fluorescence is efficiently collected in parallel across the plane by an orthogonal detection lens. The axial resolution achieved with LSM depends on the thickness of the illuminating sheet and the natural divergence of a focused Gaussian beam limits the region over which high-resolution can be achieved. Our previous research into the use of propagation-invariant beams - beams that cheat diffraction - has shown that the use of an Airy beam for light-sheet illumination can extend the usable field-of-view by over an order of magnitude compared to a standard Gaussian beam without compromising on resolution, contrast, speed, or phototoxicity [1].
I will give an overview of our recent work in this area including; the development of low-cost single- and multi-photon excitation implementations [2,3], further tailoring of the light-sheet profile to counteract intensity losses at depth, studies in neuroscience, and new sample mounting strategies.
- T. Vettenburg et al, "Light-sheet microscopy using an Airy beam", Nature Methods 11, 541-544 (2014)
- Z. Yang et al, "A compact Airy beam light sheet microscope with a tilted cylindrical lens, Biomedical Optics Express 5, 3434-3442 (2014)
- P. Piksarv et al, "Integrated single- and two-photon light sheet microscopy using accelerating beams", Scientific Reports 7, 1435 (2017)
Temporal Localized Structures in Semiconductor Lasers
Massimo Giudici (Institute de Physique de Nice) 26 April 2018, JA3.14, 11:30am
In this presentation we will describe temporal localized structures (TLS) appearing in an electrically-biased broad-area VCSEL coupled to a resonant saturable absorber mirror (RSAM). We show that the bias current is a convenient parameter for writing and erasing TLS.
Modulation of the bias current is also used to introduce a parameter landscape where statics and dynamics of TLS are analyzed. We show that their drifting speed inside the cavity depends almost exclusively on the local parameter value instead of its derivative as observed in quasi instantaneous media. This experimental observation is explained by the finite response of the semiconductor carriers which introduces causality and breaks the parity invariance inside the cavity, thus leading to a new paradigm for temporal tweezing of localized pulses inside the cavity. Different modulation waveforms are applied for describing exhaustively this paradigm. Recent experimental progress towards observation of Light Bullets will be described at the end of the presentation.
Ultrafast Plasma dynamics on Tabletop: Giant magnetic fields, Ultrahigh frequency acoustics and Shocks
G. Ravindra Kumar (Tata Institute of Fundamental Research, Mumbai) 3rd May 2018, JA3.14, 3pm
High intensity, ultrashort light pulses are revolutionizing science in exciting ways, as they can excite matter to high temperature at high density. This feature of ultrashort pulses provides a great opportunity for understanding the behaviour of matter pushed to extreme conditions pervading most of the universe! Research in this area bridges diverse areas - from astrophysics to accelerator physics and from condensed matter science to biology [1].
This talk will first introduce the subject and then dwell on the production and behaviour of 'hot' electrons (ranging up to MeV) in the plasma created by the laser. We will see results of some experiments performed at TIFR – creation of gigantic magnetic fields, ultrafast plasma dynamics, passage of relativistic particles through dense, hot matter etc.- all of which have interesting consequences in terms of electron and ion acceleration, ultrafast hard x-ray emission, laser fusion, laboratory astrophysics etc. [2-6].
- G. Ravindra Kumar, "Intense, ultrashort light and dense, hot matter" Pramana- Journal of Physics, 73, p 113-155 (2009) [Tutorial Review]
- S. Mondal et al., Phys. Rev. Lett. 105, 105002 (2010); Proc. Natl. Acad. Sci. (USA) 109, 8011 (2012)
- K.Ohta et al. Phys.Rev.Lett. 104, 055001 (2010); H. Habara et al., Phys. Plasmas 17, 056306 (2010)
- G. Chatterjee et al., Phys.Rev.Lett. 108, 235005 (2012)
- A. Adak et al, Phys. Rev.Lett 114, 115001 (2015)
- G. Chatterjee et al., Nature Commun. 10.1038/NCOMMS15970 (2017)
Elucidating the nanoscale architecture of the plasma membrane with super-resolution spectroscopy
Erdinc Sezgin (Weatherall Institute of Molecular Medicine, University of Oxford) 31st May 2018, JA5.07, 12noon
Diffusion and interaction dynamics of molecules at the plasma membrane play an important role in cellular signalling. Nanoscale mobility of lipids and proteins in the plasma membrane is highly heterogeneous. This heterogeneity gives invaluable information on the bioactivity of these molecules. Thus, it is crucial to accurately measure the diffusion dynamics of the membrane molecules.
Here, I will explain how we utilize super‐resolution STED microscopy combined with fluorescence correlation spectroscopy (STED‐FCS) to access the diffusion characteristics of fluorescently labelled lipid analogues and proteins in the live cell plasma membrane. In order to elucidate the role of cortical actin cytoskeleton, we also measure the diffusion dynamics in cytoskeleton‐free cell derived giant plasma membrane vesicles (GPMVs). Hindered diffusion of phospho- and sphingolipids is abolished in the GPMVs while transient nanodomain incorporation of gangliosides is apparent both in the live cell membrane and in GPMVs. This data underline the crucial role of the actin cortex in maintaining hindered diffusion modes of many but not all of the membrane molecules, and highlight a powerful experimental approach to decipher specific influences on molecular plasma membrane dynamics.
I will next address whether the diffusion behaviour of the proteins is a direct measure for the bioactivity. We demonstrate that canonical Wnt3 ligand of the Wnt pathway (responsible for cell proliferation and regeneration) exhibits domain-like diffusion which is influenced by specific lipids. Disturbing this domain-like behaviour is directly translated to the activity of the pathway leading to significant decrease in signalling in cells and relatedly an abrupt phenotype in zebrafish. Later, I will show how we can use super-resolution STED in combination with spectral imaging to demonstrate the heterogeneity in the membrane.
